Catalysis Science for a Carbon-Neutral Society
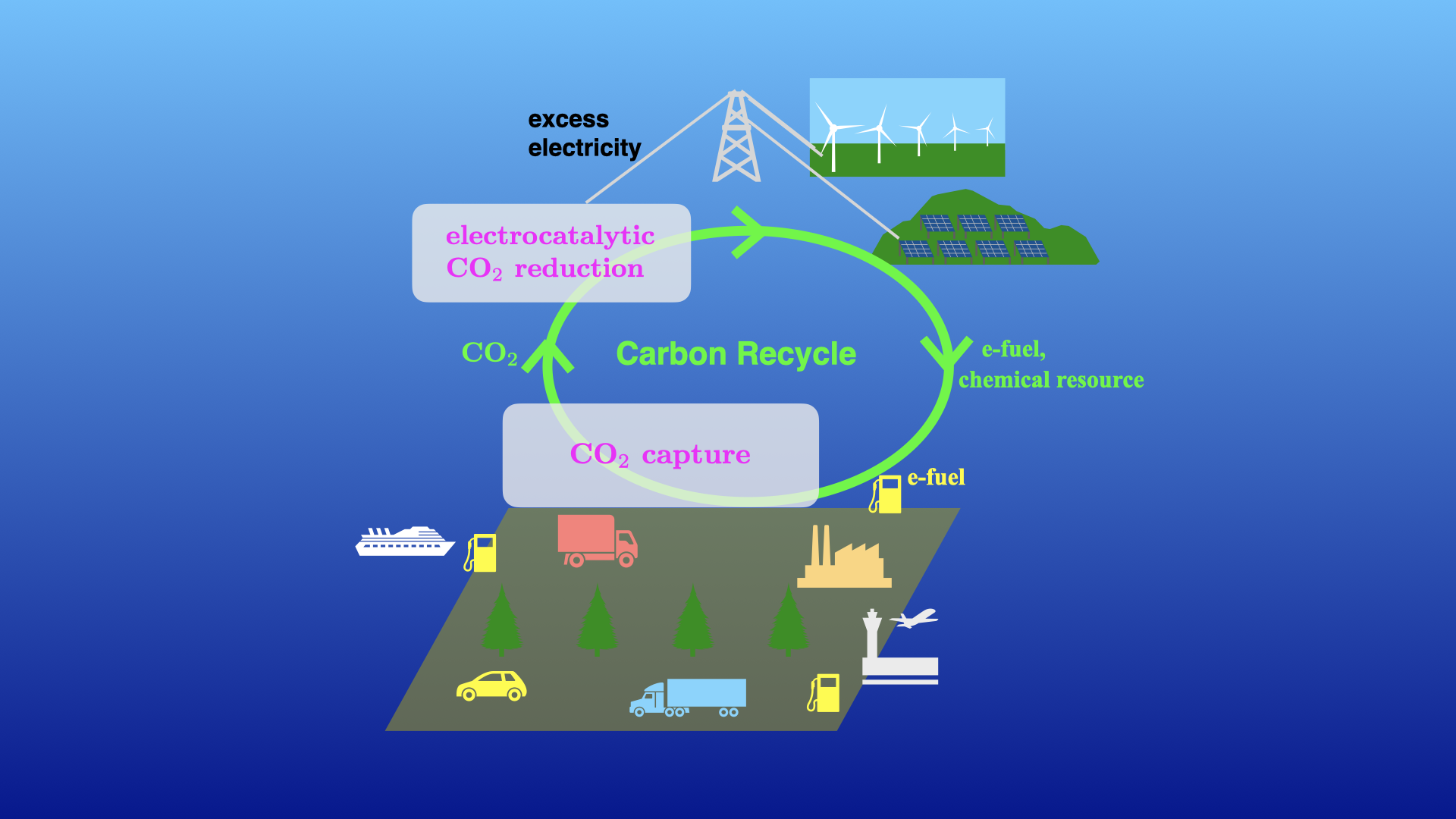
In order to realize a sustainable carbon-neutral society with low environmental impact, scientific and technological research on the capture of carbon dioxide from the atmosphere and the reduction reaction of carbon dioxide is necessary. In addition, when e-fuel created by the reduction reaction of carbon dioxide is used, purification of toxic gases is necessary, as is the case with the current use of fossil fuels.
Catalytic science is one of the key elements in the development of these sciences and technologies.
Our laboratory conducts theoretical research on chemical reactions on catalyst surfaces using first-principles calculations based on density functional theory (DFT). DFT calculations can incorporate the "individuality of catalytic reactions," so to speak, such as catalyst materials, their surface indices, and reacting and product molecules. The activation energy can also be calculated from transition state calculations using the NEB method. Furthermore, the calculation results of density of states and charge distribution in the reaction process and the analysis of the vibration frequency of adsorbed molecules provide important informations for understanding the catalytic reaction mechanism and are useful for establishing guidelines for the development of catalysts with better performance.
The reduction reaction of carbon dioxide can be achieved either by using hydrogen gas in the gas phase at high temperature or by electrochemical reactions in aqueous solution. The latter method can be operated at room temperature.It is also possible to use surplus electricity from facilities such as photovoltaic and wind power plants, which have varying generation capacities depending on the season and time of day.
Our laboratory aims to elucidate the mechanism of CO2 reduction by water on electrocatalysts. By modeling the electrocatalyst in aqueous solution as a catalyst slab and a water molecular layer, we use DFT calculations to study the electronic structure of the CO2 reduction reaction. We are also working on using machine learning, which is computationally less expensive than DFT calculations, to predict catalyst performance.